Optional Supplementary Chapter: Organic Chemistry
Organic Chemistry
Organic chemistry is a subject unto itself and began as an investigation of the chemistry of life when it was thought that only living things could create organic compounds. However, we now understand that organic chemistry is really the chemistry of carbon. Many organic molecules are rather complex and the structural formulas that are often used to describe them look simpler than the ones described above (so-called “skeletal formulas”). Here are simple rules for how to interpret a skeletal formula:
- Each vertex in a skeletal formula is a carbon atom.
- Double bonds and triple bonds are shown using two or three lines.
- Bonds that face towards the viewer are indicated with a filled triangle while bonds that face away from the viewer are indicated with striped triangle.
- Every atom that is not a carbon or a hydrogen atom is indicated explicitly at a vertex.
- Hydrogen atoms that are not connected to a carbon atom are indicated explicitly
- Hydrogen atoms that are connected to carbon atoms are omitted.
In this section, we will identify some common organic molecules that are either involved in or precursors to biochemistry. The name, chemical formula, and skeletal structural formula of each of these molecules will be indicated.
The simplest kind of organic molecule is called a “hydrocarbon”. As the name suggests, hydrocarbons are molecules that contain only hydrogen and carbon. We already considered the simplest hydrocarbon, methane, above. Simple hydrocarbon chains use methane as a starting point. These saturated chain hydrocarbons are made with only single bonds between carbon and hydrogen. Each carbon is linked to two carbons and two hydrogens unless it is on the end in which case it is linked to three hydrogens.
| ethane | C2H6 | propane | C3H8 | ||
| butane | C4H10 | hexane | C3H8 | ||
| octane | C3H8 |
It is can also be the case that organic compounds are not saturated and thus there may be double or triple bonds between carbon atoms. Here are two famous unsaturated hydrocarbons that have been detected in interstellar space.
| acetylene | C2H2 | Propylene | C3H6 |
Next, we will consider more complicated hydrocarbon structures called rings. The first ring we will consider is the cyclohexane ring. This hydrocarbon shape is very important in organic chemistry and biochemistry. It consists of six carbon atoms in a ring with the rest of the bonds taken up by hydrogen atoms. There are two conformers of the hexane ring. Known as chair cyclohexane and boat cyclohexane which are distinguished between each other by the way the bonds are oriented in the molecule. Note that cyclohexane can fairly easily flip back and forth between the two conformers which means that these are not enantiomers or chiral copies (unlike examples we will see later).
| Chair cyclohexane | C6H12 | 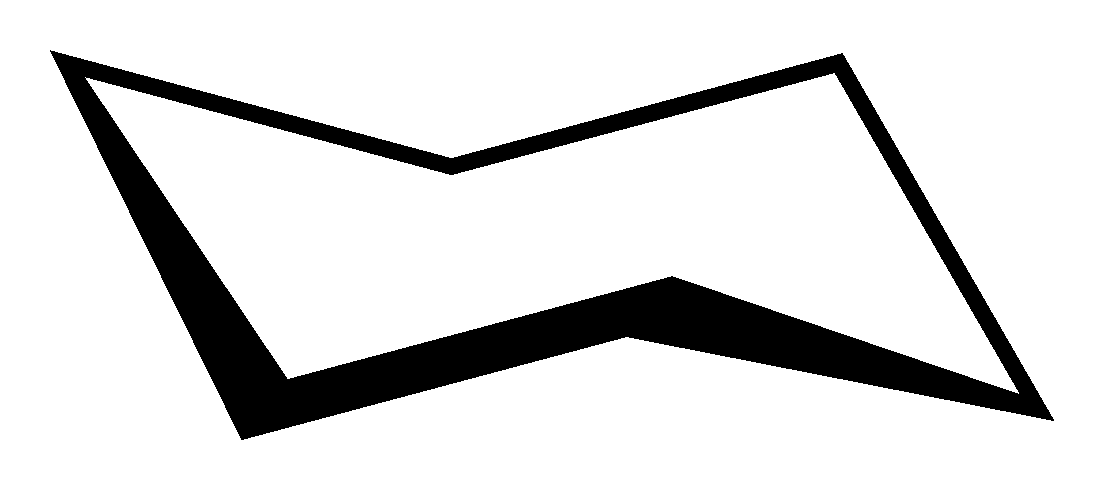 |
| Boat cyclohexane | C6H12 |  |
If instead of single bonds, half of the bonds in the ring of six carbons are double bonds, the compound benzene is formed. This hydrocarbon shape is also very important in organic chemistry and biochemistry. Moreover, benzene has been detected in interstellar space. Benzene consists of six carbon atoms that are bonded in a single bond to one adjacent carbon atom and a double bond to another. In reality, however, this alternating single-double bond configuration is only an approximation of the actual bonds that are happening which are actually something more akin to 1.5 bonds shared between every adjacent carbon atom. If you study the molecular orbitals, it becomes obvious how this can occur, but in our situation we must approximate it through alternating single and double bonds.
| benzene | C6H6 |
When comparing the two hydrocarbon rings the difference between their 3-D geometries that is most apparent is that benzene is flat or planar while cyclohexane has a three-dimensional form.
Now we will consider carbon all by itself. Carbon can form many interesting structures even isolated, and depending on how the bonds happen, there will be different properties of the resulting material. These different forms are called the allotropes of carbon. The two most common allotropes of carbon are graphite and diamond. Graphite shares many bonding features with the benzene ring but the hydrogens are replaced with carbon atoms that are connected to other rings. This forms a planar graphite structure that can explain the flakiness and flat physical properties of graphite. To form a model of the diamond structure, a modeler can start with the chair hexane ring and replace the hydrogen with carbons. Other chair hexane rings can branch off to form a sturdy and 3-D crystal structure that explains some of hardness and crystal properties of diamond. Other allotropes of carbon have been discovered as well with one of the more unique ones being fullerene where sixty carbons are bonded together to form a soccer-ball shaped molecule. There have been some spectroscopic observations that indicate this sort of molecule may be found in interstellar dust.
| graphite | C | 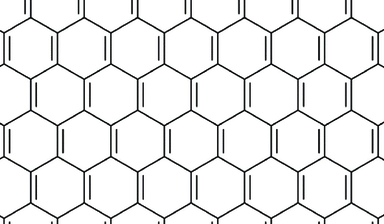 |
| diamond | C | 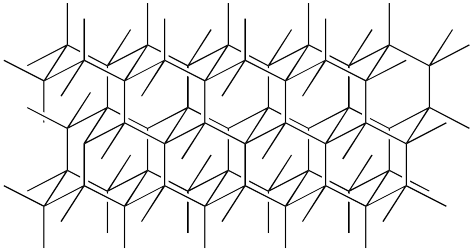 |
| fullererne | C60 |  |
Adding other atoms to the hydrogens and carbons we’ve been considering up until now greatly increases the possibilities for organic chemistry. If we consider oxygen-contained molecules as well, a new variety of organic molecules is seen. An oxygen attached to a hydrogen is called a “hydroxyl” group and is extremely important to biochemistry. The simplest hydroxyl containing organic molecules are called “alcohols” and a few have been discovered in interstellar space.
| methanol | CH3OH | ethanol | C2H5OH | ||
| ethylene glycol | C2H4(OH)2 | vinyl alcohol | C2H3OH |
An organic molecule that has a double-bonded oxygen on one end is called an “aldehyde”. The oxygen double bonded to a carbon is called a “carbonyl” group, and at least three aldehydes have been detected in interstellar space
| formaldehyde | CH2O | glycolaldehyde | C2H4O2 | ||
| propionaldehyde | C3H6O |
An organic molecule with a carbonyl group in the middle of a chain is called a “ketone” with at least two, acetone and cyclopropenone, detected in interstellar space. If there is a carbonyl group bonded to a hydroxyl group on the same carbon, the results is an organic acid. Two organic acids, formic acid and acetic acid, have been detected in interstellar space. If an organic acid and an alcohol react with each other, a molecule of water and an ester can be formed. Esters are known to produce many of the scents most easily detected by humans, and two, ethyl formate and methyl acetate, have been detected in interstellar space. Finally, if two alcohols react with each other, a molecule of water and an ether can be formed. Ethers are extremely important in biochemistry as their chemistry features in carbohydrates and biopolymers. The only ether detected in interstellar space so far has been dimethyl ether.
| acetone | C3H6O | cyclopropenone | C3H2O | ||
| formic acid | CH2O2 | acetic acid | C2H4O2 | ||
| ethyl formate | C3H6O2 | methyl acetate | C3H6O2 | ||
| dimethyl ether | C3H6O |
Now let’s add nitrogen to our organic molecules. All such molecules are based in part on the chemistry of ammonia. An amine is ammonia with one of the hydrogens replaced with a different functional group while an amide is the same except it is bonded first to a carbonyl and then to a different functional group. Two amines, methyl amine and aminoacetonitrile, and three amides, urea, methanamide, and acetamide, have been discovered in interstellar space .
| methyl amine | CH3NH2 | aminoacetonitrile | C2H4N2 | ||
| urea | CO(NH2)2 | methanamide | CHONH2 | ||
| acetamide | C2H3NH2 |
Molecules with sulfur and phosphorus have also been discovered. The three organosulfides that have been detected by astronomers are methyl mercaptan, thiocyanic acid, and thioformaldehyde while one organic compounds containing phosophorus have been identified: phosphaethyne.
| methyl mercaptan | CH4S | thiocyanic acid | CHSN | ||
| thioformaldehyde | CH2S | phosphaethyne | CHP |
Though not yet discovered in astronomical contexts, carbohydrates are important organic compounds in biochemistry and will be of interest to us for later modeling. One important class of carbohydrates are the sugars. Sugars are complicated enough that they often come in a variety of forms including some that are stereoisomers meaning that they have distinguishable characteristics from their mirror images. As described above, this feature is known as “chirality”. “Chirality” refers to the property that left and right human hands have in that the left and right are mirror images of each other, but there is no way in three dimensional space to move the right hand so that it matches the left (try it yourself!). This is the relationship that enantiomers have with each other. In general, only one enantiomer of a chiral molecule will be biochemically active. For example, certain enantiomers of sugars cannot be digested by many living things even though their chemistry is essentially the same (you would still be able to taste them as sweet, for example). For amino acids and nucleic acids that undergo interactions that depend highly upon precise “key and lock” type matching between one molecule and another, this becomes extremely important.
| glucose | ||
| sucrose | ||
| ribose | ||
