2 Virology of COVID-19: Transmission, Variants & Treatments
Unit Authored by:
- Diane Price Banks
- Grace Tursi-Wenzler
Goals:
-
To understand the virology of Sars-CoV-2, mode of transmission, the development of variant strains and list of treatment options available during the pandemic.
-
To identify how health disparities impacted the transmission and treatment of SARS-CoV-2.
Learning Objectives:
-
To understand the virology of SARS-CoV-2.
-
To examine modes of transmission and techniques used to prevent further spread.
-
To explore various developments and impact of SARS-CoV-2 variants on the spread of the virus in various countries.
-
To explore the various treatments employed during the pandemic and how health disparities may have played a role in its dissemination.
Unit Table of Contents
-
Introduction
-
Key Definitions
-
Virology of SARS-CoV-2
-
Modes of Transmission and Techniques Used to Prevent Future Spread
-
Modes of Transmission
-
Techniques Used to Prevent Future Spread
-
-
Development and Impact of the SARS-CoV-2 Variants on the Spread of the Virus in Various Countries
The outbreak of Severe Acute Respiratory Syndrome Coronavirus 2 (hereafter -SARS-CoV-2) is a zoonotic disease that began in November 2019 in Huanan Seafood Market in a small town of Wuhan, China in the Hubei Province (Pekar et. al., 2022 & Worobey et. al., 2022). The vendors at the market sold live wild and farmed animals as food and pets such as several species of mammals, birds, and reptiles (Xiao et. al., 2021) see Table 1 for a complete list. In March 2020, the World Health Organization declared SARS-CoV-2 commonly known as COVID-19 a pandemic (CDC, 2022) and President Donald J. Trump declared it a nationwide emergency as the outbreak entered the shores of the United States of America and was infecting the population. In this chapter, we will explore the virology of COVID-19, examine the modes of transmission, understand the variants and discuss available treatment options. It’s important to note that at the time of this publication the pandemic is ongoing and new information is evolving and is accurate as of July 2022.
-
Virus – a non-living infectious agent also known as a pathogen that causes disease and provokes the host immunologic response. It replicates using the infected host biological system. A virion is the term for a single viral particle.
-
Virology – the study of the biology of viruses and viral diseases, including the distribution, biochemistry, physiology, molecular biology, ecology, evolution and clinical aspects of viruses (Nature, 2022).
-
Viral Transmission – Viral transmission is the process by which viruses spread between hosts. It includes spread to members of the same host species or spread to different species in the case of viruses that can cross species barriers. (Nature, n.d. & Louten, 2016).
-
Variant – A variant is a viral genome (genetic code) that may contain one or more mutations. In some cases, a group of variants with similar genetic changes, such as a lineage or group of lineages, may be designated by public health organizations as a VBM (variant being monitored), VOC (variant of concern), or a VOI, (variant of interest), due to shared attributes and characteristics that may require public health action (CDC, n.d.).
-
Variants being monitored (VBM) – Variants designated as VBM include those where data indicates there is a potential or clear impact on approved or authorized medical countermeasures or that have been associated with more severe disease or increased transmission but are no longer detected, or are circulating at very low levels, in the United States. These variants do not pose a significant and imminent risk to public health in the United States.
-
Variant of interest (VOI) – A variant with specific genetic markers that have been associated with changes to receptor binding, reduced neutralization by antibodies generated against previous infection or vaccination, reduced efficacy of treatments, potential diagnostic impact, or predicted increase in transmissibility or disease severity.
-
Variant of Concern (VOC) – A variant for which there is evidence of an increase in transmissibility, more severe disease (for example, increased hospitalizations or deaths), a significant reduction in neutralization by antibodies generated during previous infection or vaccination, reduced effectiveness of treatments or vaccines, or diagnostic detection failures.
-
Mutation – A mutation refers to a single change in a virus’s genome (genetic code). Mutations happen frequently, but only sometimes change the characteristics of the virus (CDC, n.d.). Mutations could be silent, beneficial (makes it stronger) or harmful (makes it weaker) to a virus.
-
Portal of exit – is the path by which a pathogen leaves its host. The portal of exit usually corresponds to the site where the pathogen is localized.
-
Portal of entry – The portal of entry refers to the manner in which a pathogen enters a susceptible host.

Virology – a subfield of microbiology – is the study of viruses and the diseases they cause to include how it spreads (transmission), its genetic makeup (genetic code), its physiology, and molecular structure. A scientist who studies the biology of viruses in hopes of developing a treatment, vaccine or cure is called a virologist. Dr. Kizzmekia Corbett, an African American woman, was the key scientist behind the development of the COVID-19 vaccine produced by Moderna in partnership with the National Institute of Health (NIH) (Romero et. al., 2020). Dr. Corbett’s team developed a mRNA vaccine, the first of its kind, to target a spike protein region on the surface of the viral structure. A scientist who studies the spread of disease in a given population in the hopes of preventing or mitigating outbreaks is called an epidemiologist. During the COVID-19 pandemic, virologists and epidemiologists were hard at work understanding and sharing the virology of COVID with the world. Researchers discovered COVID-19 virus was highly sensitive to mutations and developed variants that afforded the disease a higher rate of infectivity. Mutations are changes that occur in the genetic code of an organism. Mutations occur frequently but do not necessarily result in a change in the characteristic of the virus. However, when mutations do occur and change the infectivity or characteristics of the virus then it leads to the development of new variants or in other words new or modified versions of the same virus. To better understand the virus genome and physiology, a scientist had to sequence the virus RNA and build digital models.
Let’s take a look at some digital models of SARS-CoV-2
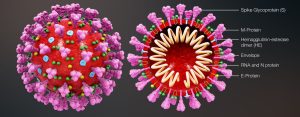
The Eckert & Higgins (2020) SARS-CoV-2 model displays the enveloped structure of the virus and its components. The SARS-CoV-2 structure consists of several spike glycoproteins (S), M proteins, hemagglutinin-esterase dimer (HE), the viral envelope, and E proteins. Inside the viral envelope are the RNA and N protein. Based on the understanding of the viral structure, Dr. Corbett and her team successfully created their mRNA vaccine to target the spike glycoprotein (S). The science of virology provides humans with the tools needed to understand the virus to prevent transmission, and develop treatments and cures. The availability of computer modeling further assists researchers with understanding the infectivity of SARS-CoV-2. Figure 2 is a computer generated model of SARS-CoV-2 as it penetrates a cell. Virologists were able to delineate the infection process in a digital format. The first image in figure 2 shows SARS-CoV-2 after it has penetrated the host’s first line of defense. As one will observe, the fusion peptide (FP) component is embedded within the structure. The FP remains hidden within the viral structure until the spike protein has attached to the host cell. Once the spike protein is attached, the FP begins to separate and migrate to the end of the spike protein to form a spearhead at the base of the attachment (ACE2 receptor) (see third image). The spearhead will pry open the host cells’ membrane. Once opened, the virion can release its genetic materials into the cells cytoplasmic space. The genetic material will then be synthesized by the host cell to eventually replicate new virion which is assembled in the cell and then released through the budding mechanism. SARS-CoV-2 is an enveloped virus that when released from the host cell will take some of the cell’s cytoplasm and cell membrane with it. (Note: naked viruses are released through lysis). This process can be simplified to A.P.U.S.A.R which stands for Adsorption, Penetration, Uncoating, Synthesis, Assembly and Release.
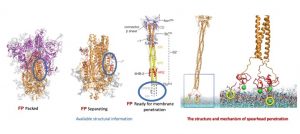
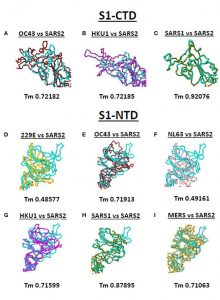
Figure 3 below depicts superimposed comparisons of SARS2 spike S1-CTD (C terminal domain) and SARS2 spike S1-NTD (N-terminal domain) with various spike-containing coronaviruses. Superimposing known coronaviruses with SARS2 allowed researchers to determine the difference in the viral structure when compared to other human infecting coronaviruses. Can you spot the differences?
Modes of Transmission:
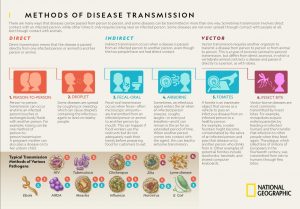
Viruses like COVID-19 are transmitted by either direct or indirect contact (see Figure 4). According to the World Health Organization (2021), the virus spreads between people in close contact with each other. Close or direct contact with COVID-19 is a conversational distance which is less than 3ft. The virus typically spreads from an infected person’s (portal of exit) mouth or nose in small liquid particles called aerosols when they cough, sneeze, speak, sing or breathe (WHO, 2021). A person within the conversational space can then contract the virus from the aerosols passed through the air by (portal of entry) inhalation (short-range airborne transmission) or when infectious particles come in direct contact with the eyes, nose, or mouth (droplet transmission).
The aerosols containing the virus spreads well in poorly ventilated and crowded indoor settings. Spending longer than 15 minutes in a poorly ventilated indoor space with infectious particles increases one’s risk of contracting the virus. With indirect contact, the aerosols can remain suspended in the air for several hours and travel farther than the conversational distance (long-range airborne transmission) (WHO, 2021).
People may also become infected via a vector when touching their eyes, nose, or mouth after touching surfaces or objects that have been contaminated by the virus (see Figure 4).
Techniques to prevent the spread of SARS-CoV2:
To help prevent the spread of SARS-CoV2, the World Health Organization recommends avoiding the “three C’s” during the pandemic; crowded places, close-contact settings, and confined/enclosed spaces (see Figure 5).

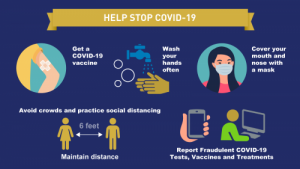
Besides avoiding the three C’s the Federal Drug Administration (FDA) recommends getting the COVID -19 vaccine which helps build antibodies in defense of the disease; proper hand washing with lukewarm water for 20 seconds often or using have sanitizer that contains at least 70% alcohol; wearing an approved face mask that covers the nose and mouth; practice physical distancing of 6ft; and reporting fraudulent COVID-19 tests, vaccine cards, etc (see Figure 6). (Office of the FDA Commissioner, 2021).
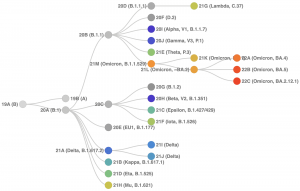
All viruses, including SARS-CoV-2, the virus that causes COVID-19, mutate genetically over time as the virus moves from host to host. As stated in section 1 of this chapter, most mutations have little to no impact on the virus’ characteristics. However, some mutations may affect the virus to afford it the ability to spread easier, increase disease severity, and decrease the performance of vaccines, therapeutic medicines, diagnostic tools, or other public health and social measures (WHO, 2022). Mutations and the development of new variants can be tracked and classified through genetic sequencing. It’s important to mention that sometimes new variants emerge and disappear as with 19B(A) (see Figure 7). Figure 7 displays the latest tracked lineage of SARS-CoV-2 variants from the wild type (original 19A(B)) strain to its variants due to mutations in its genetic coding.
The emergence of more infectious and deadly variants in late 2020 prompted the characterization of specific Variants of Interest (VOIs) and Variants of Concern (VOCs), to improve disease surveillance, research, and inform the ongoing response to the COVID-19 pandemic. “A VOI might require one or more appropriate public health actions, including enhanced sequence surveillance, enhanced laboratory characterization, or epidemiological investigations to assess how easily the virus spreads to others, the severity of disease, the efficacy of therapeutics and whether currently approved or authorized vaccines offer protection” (WHO, 2022).
As the virus spreads, it has new opportunities to mutate and may become more difficult to stop. WHO and its international network of experts constantly monitor mutations of the virus in the event significant mutations occur that greatly alter the virus characteristics ultimately resulting in variants. The U.S. Department of Health and Human Services (HHS) established a SARS-CoV-2 Interagency Group to enhance coordination among the CDC, National Institutes of Health (NIH), Food and Drug Administration, Biomedical Advanced Research Development Authority, and Development of Defense. This global and national system of monitoring affords agencies the ability to inform countries and the public about newly classified variants and any public health changes that need to occur in response to the variant to ultimately prevent its spread. “Reducing transmission through established and proven disease control methods/measures, as well as avoiding introductions into animal populations, are crucial aspects of the global strategy to reduce the occurrence of mutations that have negative public health implications” (WHO, 2022). See Figure 8 for a digital representation of the variant’s differences in size and structural characteristics.
Here’s a reference list of SARS-CoV-2 VBM and VOC to date. Use this list in conjunction with Figures 7 & 8.
- Variant Being Monitored (VBM)
- Alpha (B.1.1.7 and Q lineages)
- Beta (B.1.351 and descendent lineages)
- Gamma (P.1 and descendent lineages)
- Delta (B.1.617.2 and AY lineages)
- Epsilon (B.1.427 and B.1.429)
- Eta (B.1.525)
- Iota (B.1.526)
- Kappa (B.1.617.1)
- 617.3
- Mu (B.1.621, B.1.621.1)
- Zeta (P.2)
- Variant of Concern (VOC)
- Omicron (B.1.1.529, BA.1, BA.1.1, BA.2, BA.3, BA.4 and BA.5 lineages)
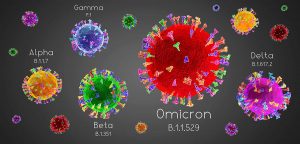
During the pandemic more scientific information became available which lead to several changes to the variant clarification list including renaming SARS-CoV-2 variants from alpha to lambda (see Figure 9). Figure 9 also includes areas where each variant was identified first and countries of prevalence.
Media Attributions
- Headshot: Dr Kizzmekia Corbett
- Figure 1. Credit: Wikimedia Commons. (Eckert & Higgins, 2020)
- Figure 2. (Weill Cornell Medical, 2021)
- Figure 3. (Cuerno and Imani, 2021)
- Figure 4. (National Geographic Society, 2022)
- Figure 5. (WHO, 2021).
- Figure 6. (Office of the FDA Commissioner, 2021)
- Figure 7. (Roemer et al., 2022)
- Figure 8. (Koppe, 2022)
