61 Hydrological Cycle, Water in Society
Water: curse or blessing?
July 4, 2025: Catastrophic Flood in Texas
-
In the early hours of July 4, floods swept through Hill Country, a region of Central Texas also known as “Flash Flood Alley.” Its propensity for high levels of rainfall combined with thin soil, exposed bedrock and steep terrain make it especially vulnerable. Rainfall gets funneled through the hilly terrain and canyons into the valleys of the region.
-
The Guadalupe River rose 20 feet in three hours. By 10 a.m., it swelled in the town of Comfort, surging to 34 feet from three feet in about 90 minutes.
-
Consequences: at least 129 fatalities and around 170 missing
-
The tragedy was particularly felt at Camp Mystic, a girls’ summer camp, where 27 campers and staff members died.
Was it human made or natural disaster?
Could we prevent it?
Eight Catastrophic Floods in 11 says:
NBC News, Sept 12, 2023

Phases of the Hydrologic Cycle, Water Use
Because of the unique properties of water, water molecules can cycle through almost anywhere on Earth. The water molecule found in your glass of water today could have erupted from a volcano. In the intervening billions of years, the molecule probably spent time in a glacier or far below the ground. The molecule surely was high up in the atmosphere and maybe deep in the belly of a dinosaur. Where will that water molecule go next?
Three States of Water
Water is the only substance on Earth that is present in all three states of matter—as a solid, liquid or gas. (And Earth is the only planet where water is present in all three states.) Because of the ranges in temperature in specific locations around the planet, all three phases may be present in a single location or in a region. The three phases are solid (ice or snow), liquid (water), and gas (water vapor). See ice, water, and clouds (figure 2).

Mysteries of Water:
https://nautil.us/five-things-we-still-dont-know-about-water-235479/#:~:text=It’s%20clear.,snow%20and%20it%20makes%20ice!
- At latest count, there are 17 different crystalline forms of solid water. However, only one form—Ice Ih—exists commonly on Earth outside of the laboratory. A second crystalline form called Ice Ic is present in very minor amounts in the upper atmosphere, and another 15 forms occur only at very high pressures. (There is also a lot of water in interstellar space, but it is usually an amorphous, non-crystalline, glassy ice frozen onto dust grains.)
- Several decades ago, Japanese scientists claimed to have observed transitions between two phases of amorphous ice under high pressure. Since we believe that amorphous ice is essentially a frozen snapshot of the corresponding liquid, this observation implied that two types of liquid water must exist: normal, low-density water, and a compact high-density form analogous to high-pressure amorphous ice.
- The rate of evaporation of liquid water is one of the principal uncertainties in modern climate modeling. It determines the size distribution of water droplets in clouds, which, in turn, determines how clouds reflect, absorb, and scatter light. But the exact mechanism for how water evaporates isn’t completely understood.
-
There is something remarkable about the mist surrounding Niagara Falls: The individual droplets move as if they are negatively charged. The same is true for most waterfalls. This has long been interpreted as evidence for the accumulation of negative hydroxide (OH-) ions at the droplet surfaces, which would mean that the surfaces are basic—with a pH value greater than the 7 of neutral water. In fact, this thinking has become dogma within the community of colloid scientists.
The surface of liquid water contains a larger number of broken hydrogen bonds, which produce a rather different chemical environment than that found in the bulk. But recent experiments and calculations suggest that hydrated protons (H+) actually dominate the liquid water surface, producing an acidic (less than 7) pH and a positively charged surface, rather than a basic, negatively charged surface.
- Water isn’t always sloshing around in giant oceans. Both in nature and in man-made devices, water is often confined to unimaginably tiny spaces, like reverse micelles, carbon nanotubes, proton exchange membranes, and xerogels (which are highly porous glassy solids).Both experiment and calculation seem to indicate that water confined by solid walls to tiny regions of space, whose size is comparable to that of a few hundred molecules, begins to exhibit quantum mechanical effects, including delocalization and quantum coherence. These properties are strikingly different from those of bulk water, and could influence everything from biological cells to geological structures. It could be also be of considerable practical significance, for example in designing more efficient desalinization systems.Current results remain somewhat ambiguous, however, and more work in this area remains to be done in order to determine the nature of water under confinement.
The Water Cycle
Because Earth’s water is present in all three states, it can get into a variety of environments around the planet. The movement of water around Earth’s surface is the hydrologic (water) cycle (figure 3).
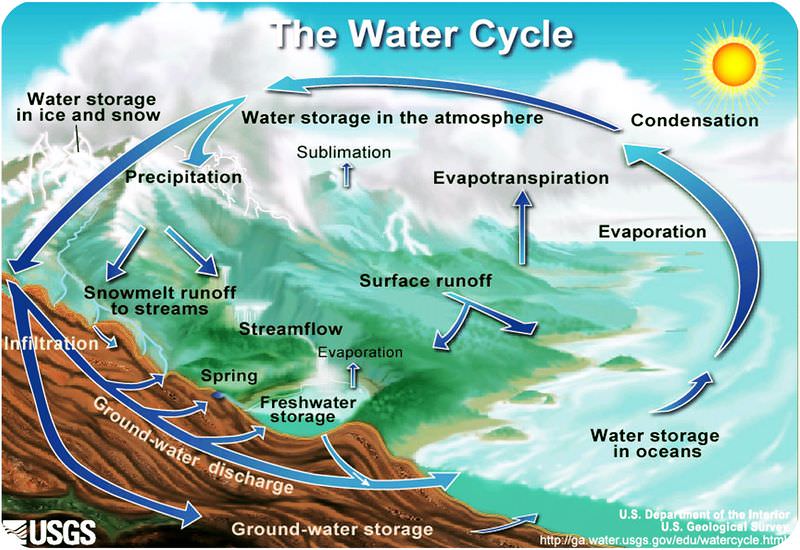
Most of Earth’s water is stored in the oceans where it can remain for hundreds or thousands of years. The oceans are discussed in detail in the chapter Earth’s Oceans.
Water changes from a liquid to a gas by evaporation to become water vapor. The Sun’s energy can evaporate water from the ocean surface or from lakes, streams, or puddles on land. Only the water molecules evaporate; the salts remain in the ocean or a fresh water reservoir.
The water vapor remains in the atmosphere until it undergoes condensation to become tiny droplets of liquid. The droplets gather in clouds, which are blown about the globe by wind. As the water droplets in the clouds collide and grow, they fall from the sky as precipitation. Precipitation can be rain, sleet, hail, or snow. Sometimes precipitation falls back into the ocean and sometimes it falls onto the land surface.
For a little fun, watch this video.
This animation shows the annual cycle of monthly mean precipitation around the world.
Satelite data blended with analytical models and algorithms can help to build global precipitation maps, such as: https://sharaku.eorc.jaxa.jp/GSMaP/ This global map is based on satellite mission Global Precipitation Measurement.
When water falls from the sky as rain it may enter streams and rivers that flow downward to oceans and lakes. Water that falls as snow may sit on a mountain for several months. Snow may become part of the ice in a glacier, where it may remain for hundreds or thousands of years. Snow and ice may go directly back into the air by sublimation, the process in which a solid changes directly into a gas without first becoming a liquid. Although you probably have not seen water vapor sublimating from a glacier, you may have seen dry ice sublimate in air.
Did you ever think that surface water can influence earth crust? If not, read this story:
Snow and ice slowly melt over time to become liquid water, which provides a steady flow of fresh water to streams, rivers, and lakes below. A water droplet falling as rain could also become part of a stream or a lake. At the surface, the water may eventually evaporate and reenter the atmosphere.
A significant amount of water infiltrates into the ground. Soil moisture is an important reservoir for water (figure 4). Water trapped in soil is important for plants to grow.
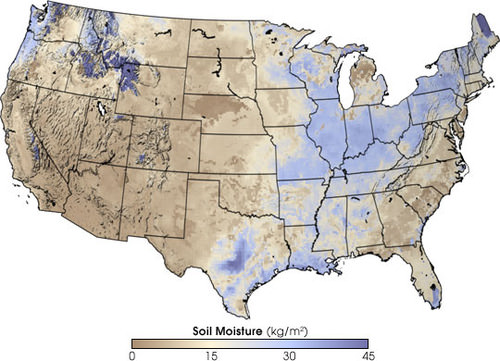
Water may seep through dirt and rock below the soil through pores infiltrating the ground to go into Earth’s groundwater system. Groundwater enters aquifers that may store fresh water for centuries. Alternatively, the water may come to the surface through springs or find its way back to the oceans.
Plants and animals depend on water to live and they also play a role in the water cycle. Plants take up water from the soil and release large amounts of water vapor into the air through their leaves (figure 5), a process known as transpiration.
An online guide to the hydrologic cycle from the University of Illinois is found here.
People also depend on water as a natural resource. Not content to get water directly from streams or ponds, humans create canals, aqueducts, dams, and wells to collect water and direct it to where they want it (figure 6).

More on ancient water technologies: Dr. Mays compilation of photos: https://ancientwatertechnologies.com/
In low-income countries like Uganda children go on average 3 – 5 hours a day to fetch water supply for the family.
(photo by Yuri Gorokhovich, Uganda, Butaleja County, 2010)
Voting for Water in India
Where is water?
https://www.youtube.com/watch?time_continue=12&v=b1f-G6v3voA
| Table 1. Water Use in the United States and Globally | ||
|---|---|---|
| Use | United States | Global |
| Agriculture | 34% | 70% |
| Domestic (drinking, bathing) | 12% | 10% |
| Industry | 5% | 20% |
| Power plant cooling | 49% | small |
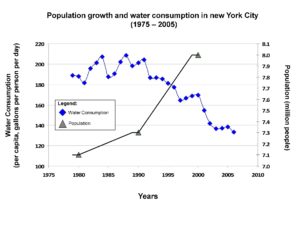
New York City: an example of sustainable water supply system
http://www.nyc.gov/html/dep/html/drinking_water/index.shtml
New York City Water Supply System (map): https://www1.nyc.gov/assets/dep/downloads/pdf/water/drinking-water/water-supply/nyc-water-supply-system.pdf Research shows that with growing population trend water demand can go down if using conservation measures
(Graph is adapted from: Gorokhovich Y., V. Goldsmith. 2009. Water Supply Management of The New York Metropolitan Area and Future Challenges From Population Growth and Global Climate Change. In: The Role of Hydrology in Water Resources Management. Ed: Hans-Jurgen Liebscher. (Proceedings of a workshop held on the island of Capri, Italy, October 2008). International Association of Hydrologic Sciences (IAHS) Publication. 327, ISBN 978-1-901502-94-7 )
What is going on with Salt Lake?
Water levels in October fell to the lowest levels on record, exposing much of the lakebed and creating conditions for storms of dust — laden with toxic metals — that now threaten the 2 million people living nearby.